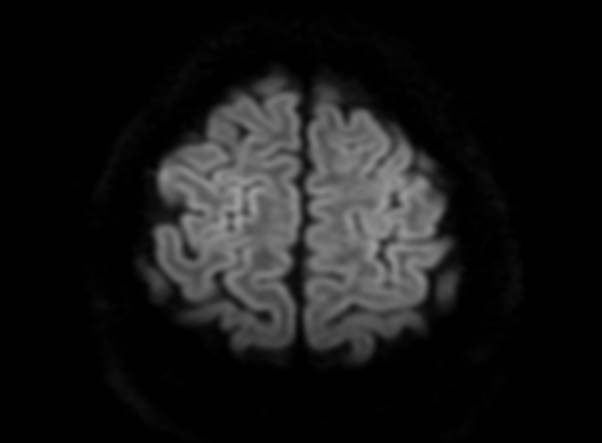
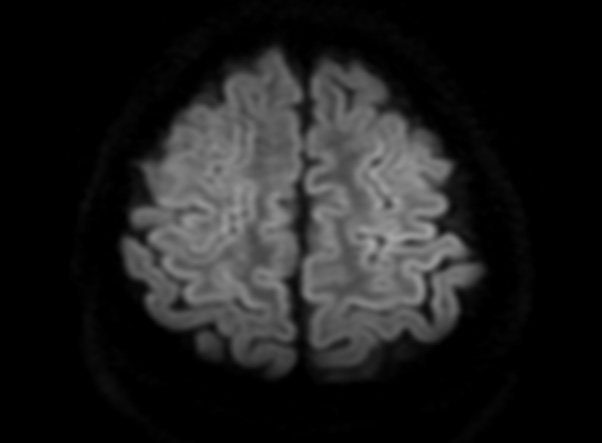
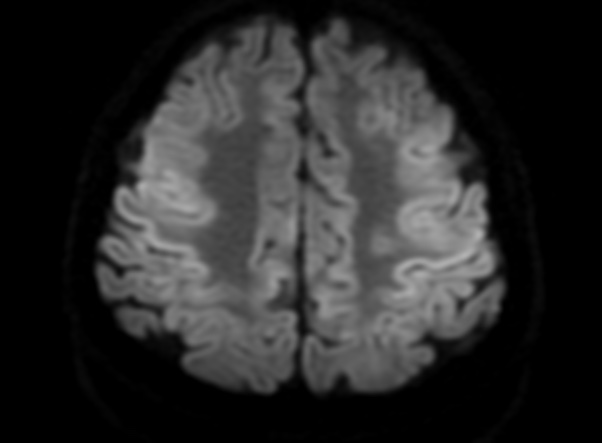
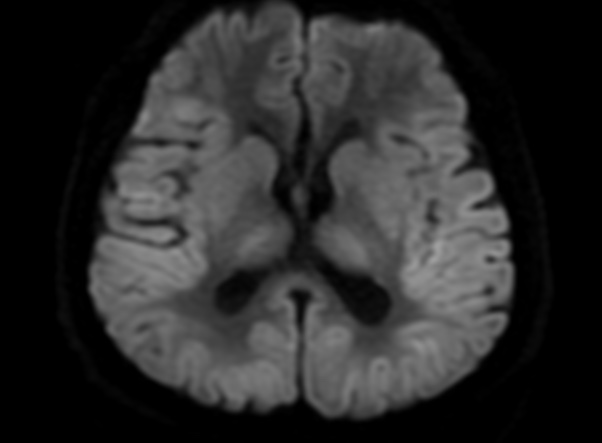
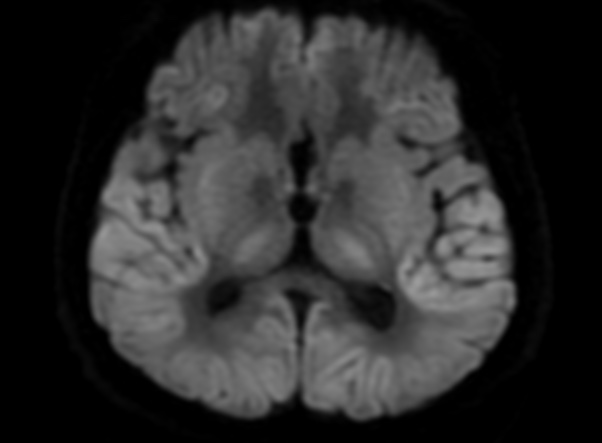
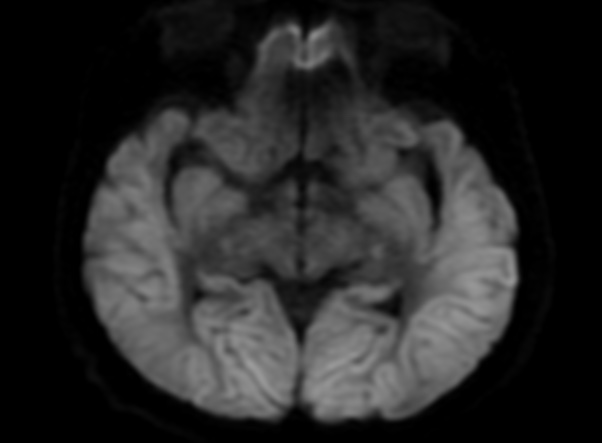
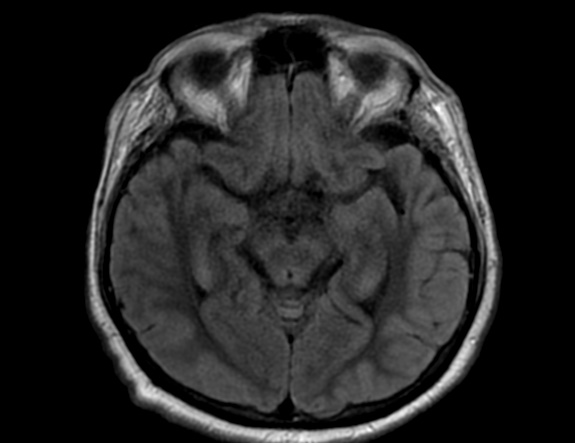
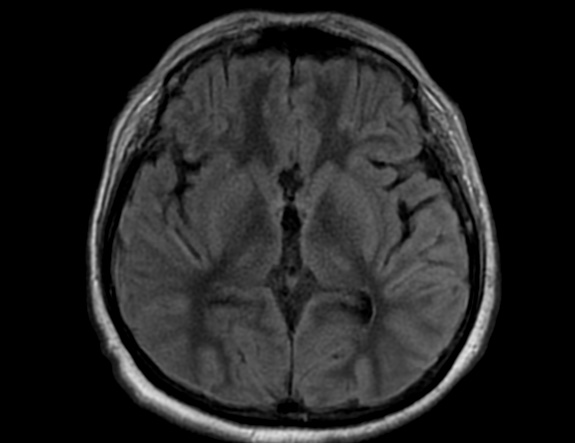
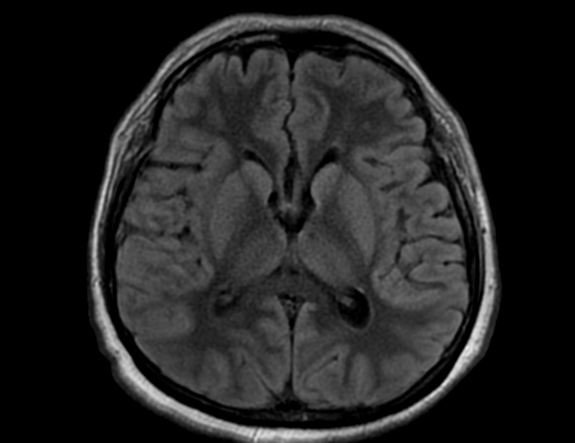
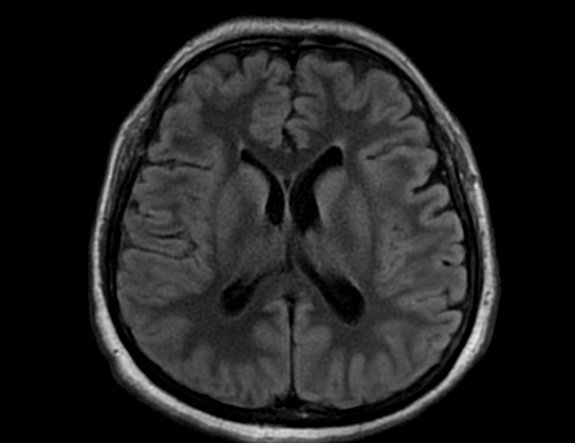
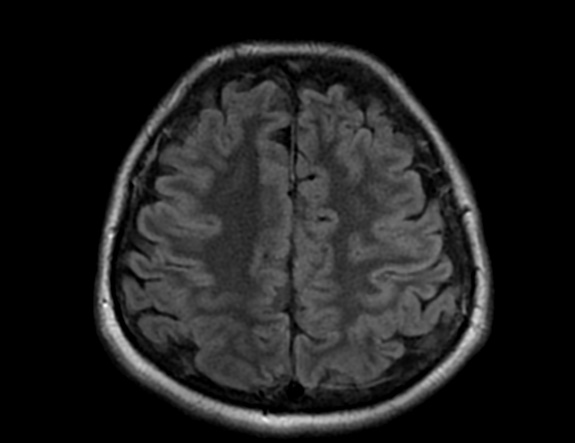
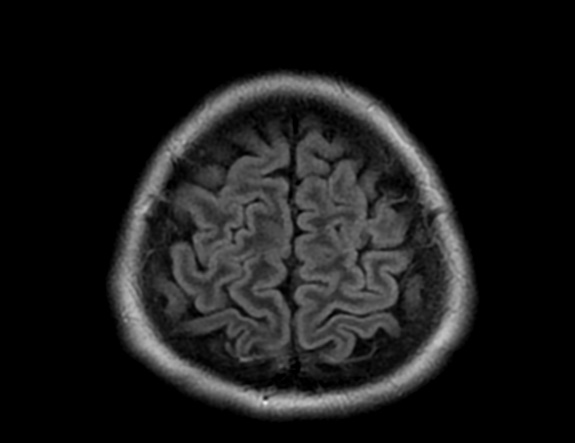
FLAIR image show symmetric
hyperintense lesions in the parietal cortices bilaterally.
Diabetic ketoacidosis with prolonged hyperglycemia may cause subtle FLAIR and diffusion abnormalities in the cortex associated with elevations in glucose, myoinositol, taurine, and ketones in MR spectroscopy.
Diabetes mellitus type 1 (DM1) is one of the most common chronic pediatric illnesses. Diabetic ketoacidosis (DKA) is serious complication in children with DM1 and has an increasing incidence. DKA is a state of severe insulin deficiency, either absolute or relative, resulting in hyperglycemia, ketonemia, acidemia, and systemic inflammation.
DKA is a rather common presentation or complication of pediatric DM1 patients and occurs in about 36% of diabetic children younger than 5 years of age and about 16% of teenagers older than 14 years. In the majority of pediatric patients, DKA has an excellent prognosis. In about 1% of the children DKA results in acute morphologic and functional brain changes that are associated with poor long-term neurocognitive outcome. Neuroimaging plays a key role in the early diagnosis of diabetic children with DKA and acute CNS involvement. Accordingly, it important that neuroradiologists are aware of the possible neuroimaging findings associated with DKA.
Cerebral edema is the most common neuroimaging finding associated with pediatric DKA and occurs in about 0.5%–1% of pediatric DKA.
Cerebral edema in DKA is associated with increased morbidity and mortality in up to 50% of affected children and occurs almost exclusively in pediatric patients. Children with more severe acidosis, hypocapnia, and dehydration have a higher risk of developing cerebral edema. The exact pathomechanism of cerebral edema in DKA, however, remains unclear and is most likely multifactorial. Possible pathomechanisms include (1) osmotic gradients drawing fluid into hypertonic astrocytes during intravenous therapy, (2) intracellular accumulation of sodium and water as a correction of intracellular acidosis due to the accumulation of lactate, free fatty acids, and ketone bodies, and (3) vasodilatation and reperfusion following cerebral vasoconstriction due to hypoocapnia.
On CT and MRI, cerebral edema in children with DKA is characterized by effacement of the sulci and basilar cisternal spaces (especially the suprasellar, quadrigeminal plate and ambient cisterns), compression and decreased size of the cerebral ventricles, and reduction of the gray-white matter differentiation.
Diffusion-weighted and diffusion-tensor imaging may further characterize cerebral edema and differentiate between vasogenic and cytotoxic edema. Increased ADC values representing vasogenic edema have been shown in the basal ganglia, thalamus, frontal white matter, and periaqueductal gray matter of children with DKA and cerebral edema. Increase in ADC values correlates with the degree of dehydration and hyperventilation at presentation, but not with factors related to initial osmolality or osmotic changes during treatment.These findings suggest that cerebral hypoperfusion may be a key role in the pathomechanism of cerebral edema in DKA. The role of cerebral hypoperfusion is supported by perfusion-weighted imaging studies showing shorter mean transit times and higher peak tracer concentrations, again suggesting vasogenic edema.
Severe cerebral edema may cause complications with high morbidity and mortality such as focal stroke and subfalcine or transtentorial herniation. Focal infarction secondary to cerebral edema may occur in up to 20% of children with DKA and typically involves the mesial basal ganglia, thalamus, periaqueductal gray matter, and dorsal pontine nuclei. Brain herniation is an uncommon complication of cerebral edema in DKA that typically occurs 6–13 hours after onset of symptoms and is clinically characterized by sudden deterioration of consciousness, absent brainstem responses, and eventual respiratory arrest. Compression of the major vessels of the circle of Willis due to downward herniation may play an additional significant factor in the occurrence of focal ischemic strokes.
Other possible neuroimaging findings in DKA include ischemic and hemorrhagic stroke, sinovenous thrombosis, and extrapontine myelinolysis. Ischemic stroke in DKA may occur independently from cerebral edema and be due to increased blood viscosity and thrombosis due to hyperosmolality, systemic inflammatory reaction, vascular endothelial injury, acidemia-induced red blood cell rigidity, and hyperglycemia-induced vasoconstriction. Hemorrhagic stroke is less common in acute DKA and may be secondary to sinovenous thrombosis.
In addition, hyperglycemia and acidosis could enhance endothelial damage with subsequent extravasation of red blood cells through the leaky vessels and increase the risk of hemorrhagic transformation of ischemic strokes.
Finally, extrapontine myelinolysis is a very rare complication. Extrapontine myelinolysis is characterized by T2-/FLAIR-hyperintense signal and restricted diffusion in the claustrum and putamen with inconsistent involvement of the hippocampi and subcortical white matter and may be associated with pontine myelinolysis. The exact pathomechanism of extrapontine myelinolysis in DKA is unknown, but is most likely related to electrolyte imbalance in the context of DKA and its rapid correction.
Reference: